Read This Before Taking Damaged Tablet – Common Tablet Defects Seen by End Users
Aug 20, 2025
Pharmaceutical tablets are one of the most widely used dosage forms for medication delivery across the globe. They offer convenience, precision in dosing, and stability. However, tablet defects can sometimes make their way to the end user—posing not only a quality concern but also potentially impacting the effectiveness and trust in the product.
While many tablet defects originate during the compression stage of manufacturing, some can manifest or become apparent during packaging, transportation, or handling. In this article, we explore the most common tablet defects observable by end users, many of which stem from issues at the compression stage but become noticeable only once the product is in the hands of the consumer.
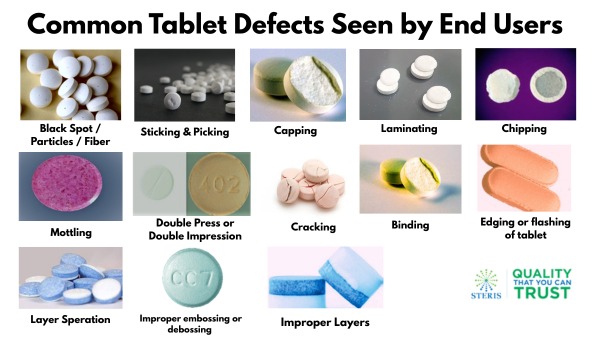
1. Black Spots / Particles / Fibers - These are unwanted dark-colored spots, fibers, or particulate matter visible on or in the tablet.
Cause -
-
Contamination from equipment (e.g., oil, grease, or metal shavings).
-
Environmental contamination (dust, fibers).
-
Raw material impurities.
Impact -
-
Compromises visual appeal.
-
May signal contamination—leading to distrust or regulatory concerns.
2. Sticking and Picking - Material adheres to the punch faces during compression. "Picking" refers specifically to material sticking in the embossing area.
Cause -
-
Poor formulation (sticky ingredients).
-
Inadequate lubrication.
-
Improper punch design or finish.
Impact -
-
Tablets appear with missing portions or logos.
-
Impaired dose uniformity.
3. Capping - Partial or complete separation of the top (or bottom) part of the tablet horizontally.
Cause -
-
Air entrapment during compression.
-
Low moisture content.
-
Poor binding properties of the formulation.
Impact -
-
Physical instability.
-
May cause dose variation or breakage during handling.
4. Laminating - The tablet splits into multiple layers.
Cause -
-
Rapid decompression.
-
Weak binding agents.
-
Inadequate compression force.
Impact -
-
Structural weakness.
-
Difficult to swallow.
-
Risk of inaccurate dosing.
5. Chipping - Breaking of tablet edges during handling or packaging.
Cause -
-
Brittle tablet formulation.
-
Excessive compression force.
-
Poor mechanical strength.
Impact -
-
Loss of tablet mass.
-
Reduced product integrity.
6. Mottling - Uneven distribution of color, giving a blotchy or spotted appearance.
Cause -
-
Uneven dye distribution.
-
Non-uniform granulation.
-
Incompatible excipients.
Impact -
-
Visual inconsistency.
-
May alarm users despite therapeutic quality.
7. Double Press or Double Impression - Tablet bears duplicate embossing or logo marks.
Cause -
-
Punches not resetting correctly.
-
Mechanical issues with the turret.
Impact -
-
Aesthetic defect.
-
Potential confusion about dosage.
8. Cracking - Fine cracks or splits visible on the tablet surface.
Cause -
-
Rapid expansion during decompression.
-
Excessive hardness.
-
Inadequate plasticity of granules.
Impact -
-
May crumble over time.
-
Raises concerns about stability.
9. Binding - Tablets are difficult to eject, causing surface damage or sticking.
Cause -
-
High friction between die wall and tablet.
-
Inadequate lubrication.
Impact -
-
Visual roughness.
-
Increased wear on machines.
10. Edging or Flashing - Thin layer or film forming around the tablet edge.
Cause -
-
Misalignment of punches.
-
Overfilled dies.
Impact -
-
Sharp edges or tablets uncomfortable to swallow.
-
Breakage risk.
11. Layer Separation - Different colored layers (in multi-layer tablets) separate.
Cause -
-
Poor bonding between layers.
-
Incompatible formulations.
Impact -
-
Dosage inconsistency.
-
Reduced therapeutic effect.
12. Improper Embossing or Debossing - Blurry, shallow, or unreadable text/logo on tablets.
Cause -
-
Worn-out punches.
-
Low compression force.
-
Sticking to the emboss area.
Impact -
-
Identification issues.
-
Regulatory non-compliance.
13. Improper Layers - In multi-layered tablets, the layers may be misaligned or unequally formed.
Cause -
-
Machine synchronization errors.
-
Variability in formulation flow.
Impact -
-
May confuse end users.
-
Risk of incorrect dosage timing (especially in modified-release tablets).
Why These Defects Matter to the End User
From a patient's perspective, any visible defect—even if it doesn’t compromise efficacy—can reduce trust and adherence. Additionally, in regulated environments, such defects may lead to recalls, investigations, and damage to brand reputation.
Final Thoughts
Tablet defects are not just a manufacturing concern—they're a critical quality issue with direct impact on patients and healthcare professionals. While many of these arise during the compression stage, their visibility at the user end underlines the need for stringent quality assurance at every stage: formulation, compression, coating, and packaging.
As a leading name in pharmaceutical quality assurance, Steris Healthcare ensures strict compliance with GMP standards and advanced technologies in inspection, detection, and process optimization—minimizing the occurrence of such tablet defects and safeguarding consumer health.
Recent Post

Why Multivitamin and Multimineral Tablets Are Essential for Daily Health and Energy Support.
Mometasone Nasal Spray : A Trusted Solution for Daily Allergy Relief
Sitagliptin 100 mg, Metformin SR 500 mg & Pioglitazone 15 mg in Triple Diabetes Therapy
Baclofen Sustained Release 30 mg: Benefits, Uses, and Safety Tips

EUROSOFT Light Moist Cream: The Secret to Daily Hydration and Soft Glowing Skin
Trazodone Hydrochloride 50 mg & 100 mg – Effective Relief for Depression, Anxiety & Sleep Issues
Bilastine 2.5mg Oral Solution – Pediatric Allergy Relief Syrup
N-Acetyl L-Cysteine (NAC), Pine Bark Extract & Resveratrol Tablets: Ingredients and Health Benefits
Remogliflozin Etabonate 100 mg: Benefits, Uses & Safety Guide
Rosuvastatin 40mg & Ezetimibe 10mg: Effective Support for Healthy Cholesterol